RehabEmbed: ReInHand Android App
Improving Hand Function of Severely Impaired Chronic Hemiparetic Stroke Individuals Using Task-Specific Training With the ReIn-Hand System: A Case Series
Front Neurol. 2018; 9: 923. Published online 2018 Nov 7. doi: 10.3389/fneur.2018.00923
PMCID: PMC6234834PMID: 30464754
Introduction
The purpose of this study is to determine the effect of device-assisted task-specific training on hand motor function and sensation (stereognosis and cutaneous sensory touch threshold) in individuals with chronic stroke and severe UE impairment.
Methods
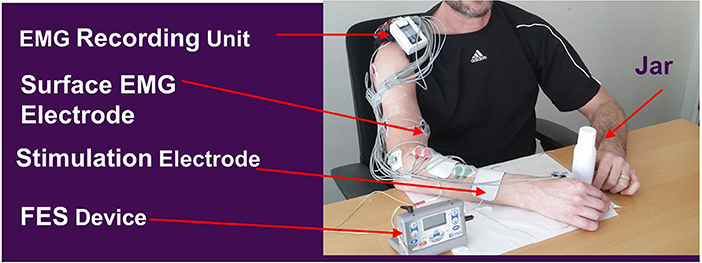
Devices
ReIn-Hand is a recently developed EMG-FES device that uses the combination of an EMG collection unit (Avatar physiological recorder, Electrical Geodesics, Inc., Eugene, OR, United States), an intelligent detection software the ReIn-HAND platform, and an electrical stimulator (Empi 300, Vista, CA, United States). The ReIn-Hand platform wirelessly and simultaneously measures surface EMG activities from eight upper limb muscles, including deltoid, biceps brachii, triceps, extensor communis digitorum, extensor carpi radialis, flexor digitorum profundus, flexor carpi radialis, and abductor pollicis. The device uses subject-dependent coherence-based notch filter to increase the signal-to-noise ratio of the collected EMG signals; it then uses the mean absolute value, zero crossing, slope sign changes, waveform length values to perform real-time detection of hand opening with or without activation of the shoulder/elbow muscles during functional upper limb motor tasks. Once hand opening is detected, a signal is sent to trigger the electrical stimulator to assist paretic hand opening. In all the subjects, including those with abnormal synergistic muscle activity and spasticity, the average detection accuracies were >90%. The stimulation electrodes were placed over finger/wrist extensors; the stimulation was set with the following parameters: amplitude sufficient for maximal hand opening without discomfort, biphasic waveform, frequency 50 ± 20%, 300 μs pulse width, and duration time of 3s.
Conclusions
These results suggest that using the ReIn-Hand device during functional reaching and grasping activities may contribute to improvements in gross motor function and stereognosis sensation of the paretic arm in individuals with moderate to severe impairment following chronic stroke.
Clinical Messages
- Task-specific training aided by the ReIn-Hand device might improve motor and sensory function in severely impaired chronic stroke.
- Further research is needed to assess the effectiveness of this intervention for improving clinical outcomes in randomized controlled trials.
About technology advancements and installation steps
Hardware Updates
In light of ongoing technological advancements, we have incorporated the BITalino MuscleBIT bundle for electromyography (EMG) signal acquisition. This bundle is tailored for individuals seeking to measure muscle activity through the evaluation of EMG signals. 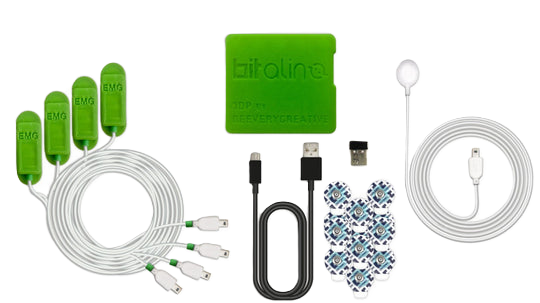 Furthermore, we have customized GPIO output ports on this hardware, aligning the trigger signal for electrical stimulators with the EMG reception signal. Both signals are transmitted via Bluetooth, establishing a wireless connection that seamlessly integrates control devices (such as tablets or smartphones) with the acquisition and stimulation devices.
Furthermore, we have customized GPIO output ports on this hardware, aligning the trigger signal for electrical stimulators with the EMG reception signal. Both signals are transmitted via Bluetooth, establishing a wireless connection that seamlessly integrates control devices (such as tablets or smartphones) with the acquisition and stimulation devices.
A Step-by-Step Guide to Software Installation
- Open https://github.com/Achillesy/Android_ReInHandv3/releases
- Download
Dr-ReIn-Hand.apkReIn-Hand.apkandreinhand.zip - Open Settings -> Connections -> Bluetooth and pair BITalino
- Open My Files -> Downloads
- Install
Dr-ReIn-Hand.apk - Install
ReIn-Hand.apk - Move
reinhand.zipto the Internal storage and Extract to current folder - Test
Dr-ReIn-Hand.apk - Test
ReIn-Hand.apk
One Bluetooth device cannot connect to multiple apps at the same time, when testing, be sure to completely close the running Dr.ReInHand or ReInHand first.
Version Control
Patient v3.11.10 & Clinician v3.11.10 Latest
1
2
3
4
5
6
7
8
9
10
11
android {
compileSdkVersion 34
defaultConfig {
applicationId "edu.northwestern.feinberg.clinician"
minSdkVersion 29
targetSdkVersion 33
versionCode 31110
versionName "3.11.10"
}
}
Tested successfully on SAMSUNG Galaxy Tab A8 10.5” Android 12
Patient v3.1.3rts & Clinician v3.1.3x
1
2
3
4
5
6
7
8
9
10
11
android {
compileSdkVersion 29
buildToolsVersion "29.0.2"
defaultConfig {
applicationId "edu.northwestern.feinberg.clinician"
minSdkVersion 27
targetSdkVersion 29
versionCode 310
versionName "3.1.3x"
}
}
Tested successfully on SAMSUNG Galaxy Tab S6 Lite Android 10